They’re among the most terrifying words a person can ever hear: “You have a brain tumor.”
But thanks to a revolutionary new technology called GammaTile®, doctors could soon accompany such grim pronouncements with the following reassurance: “But there’s reason to be hopeful.”
In technical terms, GammaTile employs a type of brachytherapy – localized radiation therapy utilizing inserted seeds, ribbons or capsules to target radiation near a cancerous growth (or after surgery to target residual malignancy). In everyday terms, it’s nothing short of a miracle.
Brachytherapy has been successfully employed for years to treat certain cancers – especially prostate cancer. In the operating room, a radioactive implant is inserted directly into the target – sometimes after surgical removal.
“The idea is that when you have a disease where surgery is potentially helpful, but you can’t take everything out, and you want to treat the edges, brachytherapy is a good way to directly deliver focused radiation right there,” explained Johnathan Engh, MD, FAANS. “Unfortunately, in the brain, once you remove the tumor, in most cases you’re still not done with it.”
Dr. Engh is a neurosurgeon at Lexington Brain and Spine Institute and specializes in awake craniotomy, minimally invasive port surgery for brain tumors, stereotactic radiosurgery and spinal tumor removal, among other procedures. He also has a special expertise in the treatment of gliomas, metastases, colloid cysts and intraventricular tumors.
“Metastases (a secondary malignant growth) are common in brain tumors, so the tumor isn’t just the tissue itself,” Engh explained. “In the old days, those people got whole radiation treatments where the entire head received radiation, including the healthy brain tissue. And that clearly has cognitive and other side effects.”
Brachytherapy came along several decades ago and changed everything. Now GammaTile is taking this approach to a whole new level. Approved by the U.S. Food and Drug Administration (FDA) in 2018 for use in treating recurrent brain tumors, GammaTile was granted additional FDA clearance for treating newly diagnosed malignant tumors in 2020.
This relatively new product is implanted into the brain at the conclusion of surgery.
“They are very small squares, one centimeter in diameter,” Engh said, “They’re placed along the edges of the cavity after the tumor is removed. They allow the radiation to work right into the edges of where you’ve operated, to try to prevent relapses of the tumor.”
With GammaTile, radiation treatment is delivered at the site from inside the brain itself, directed at areas that are most at risk while also protecting healthy tissue. Insertion usually only takes around five minutes, and is far less involved than typical radiation treatments.
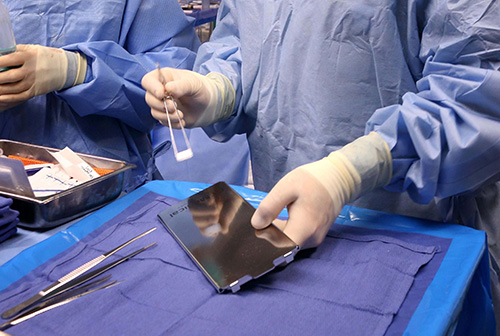
“It’s very safe,” Engh noted. “For some radiation treatments, you have to utilize people wearing protective suits and holding a Geiger counter. With GammaTile, I don’t have to wear special protective gloves to use it, just my regular surgical gloves.”
“The best candidates would be patients who have a single brain metastasis or limited burden of brain metastases that require surgery,” Engh continued. “The most common tumors that we’ll see in that scenario are lung cancers, breast cancers, melanomas and kidney cancers. Those would be the biggest indications. So this is a very good treatment option for people who must have surgery for brain tumors that are not completely curable with surgery. And that’s certainly the majority of adults.”
GammaTile radiation goes to work almost immediately, with 50 percent of its dosage being released within the first 10 days. By six weeks, more than 95 percent of its dosage has been delivered.
After the operation, the device does not need to be surgically removed at a later date. The tile dissolves over time and is absorbed into the body. There is a cosmetic benefit to the treatment, too, as just one out of 74 patients who received the implant reported experiencing hair loss.
GammaTile was implanted in its 1,000th patient nationally just last month. Also, Lexington Medical Center recently received approval to use the device – making it one of only a handful of medical facilities in South Carolina authorized to perform the treatment.
So while GammaTile may be the new kid on the medical block right now, it’s not likely to stay that way once word of its many advantages begins to spread.
“The expectation is that it’s going continue to grow,” Engh said. “For all the people who need the operation, who need the surgery, there are a lot of aspects of GammaTile that are more attractive than having to wait and do radiation after the surgery.”

Johnathan Engh, MD, FAANS, Lexington Brain and Spine Institute




Leave a comment